Write a Balanced Equation to Represent the Reaction Shown.
N 2 O 2 N 2 O 5 unbalanced N 2 O 2 N 2 O 5 unbalanced Next count the number of each type of atom present in the. Na s H 2 O l NaOH aq H 2 g Solution Begin by counting each kind of atom on both sides of the arrow.
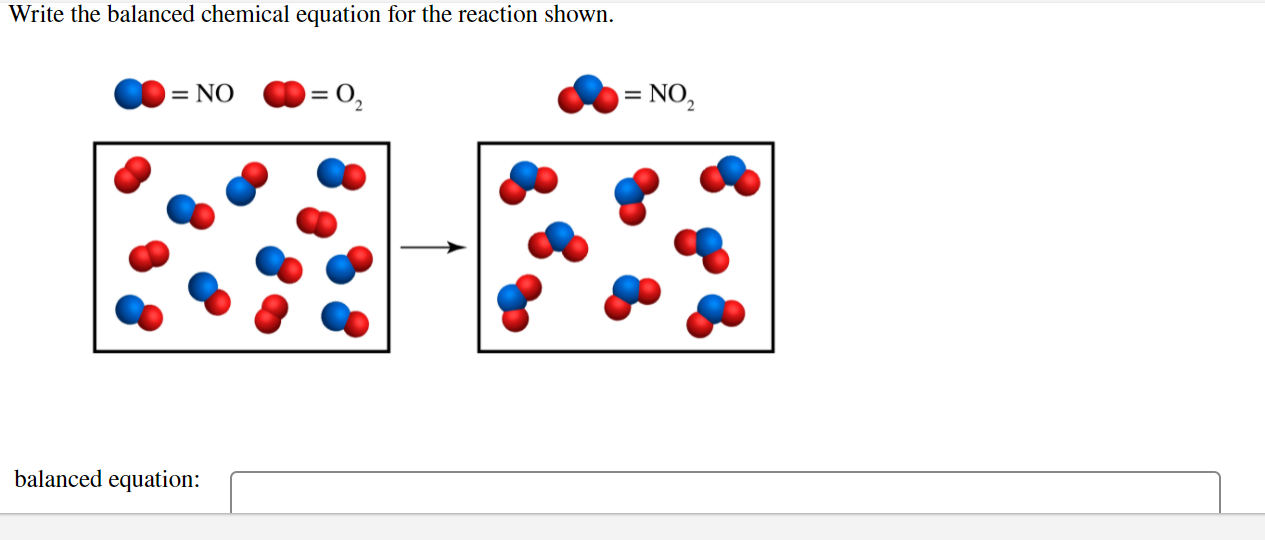
Solved Write The Balanced Chemical Equation For The Reaction Chegg Com
HOH HT H H-C-s C-H H H s-H balanced equation.

. CHS HO CHS HO. 3CH4 3 N2Cl4 ----- 3 CCl4 3N2 6H2. First write the unbalanced equation.
1 2 2 1 4. This is what I have so. 3 molecules of CCl4 3 molecules of N2 and 6 molecules of H2.
Write a balanced chemical equation including the state symbols. Write a balanced equation to represent the reaction shown. Ions with the.
Y two plus six X. Single like this hydrazine react with di di nitrogen. Convert the chemical names into chemical formulas.
Figure 1110 depicts an equation with squares representing Element M and circles representing Element N. Why do leads to formation of six X. Ionic charges are not yet supported and will be ignored.
A balanced equation that represents an overall cell reaction is shown. 4 4 yes. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen.
Molecular Complete Ionic. We review their content and use your feedback to keep the quality high. 7 Write a balanced equation to represent the reaction shown in the figure.
They are called the reactants. Byproduct is nine B two C molecules along with 68 atom. 2N 6C --3NCIA 2N601.
This is the balanced chemical equation because both A B and C atoms are equal on the product and the reactant side of the equation. The balanced equation will appear above. This side represents the substances we have beforethe reaction takes place.
That is represented as. They send for nitrogen dress They sent for oxygen and white. Write a balanced equation to represent the reaction shown in the figure.
For example when hydrogen gas reacts with oxygen gas to form water we can write a word equation for the reaction as follows. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. They send full hydrogen and therefore you can write a question in this element.
Choose the cell notation which corresponds to the electrochemical cell that is described by this equation. SCI--3NCI NACI-2NCI 8 Match the type of chemical equation with the formula 0. 2H2O1 O2g 4Haq 4e 2H2O1 2e H2g 2OHaq E 082V 14V with overvoltage oxidation E -042V -1V with overvoltage reduction.
Identify reactants and products and place them in a word equation. Fe Au Co Br C O N F. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
Place them based on the chemical equation and write the state symbols. 2N 6C --3NCIA 2N601. Write a balanced equation for the reaction of molecular nitrogen N 2 and oxygen O 2 to form dinitrogen pentoxide.
-4NCI N601 ANCI 2N. Write a balanced equation to represent the reaction shown. Writing balanced chemical equations study guide by Natara_Hill includes 22 questions covering vocabulary terms and more.
A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Cl-a B B-C1 B B B Cl-ci B-ci CIB C- сі C-8. Choose A balanced equation that represents an overall cell reaction is shown.
2 2 4. They This is unbalanced equation. -4NCI N601 ANCI 2N.
HSH нон не н-c-ss H ноор о HH нон. A Sn2 aq 71332 results Chemistry. The chemical equation or the chemical reaction will be represented as six baby three plus mind carbon Leads to formation of nine B to C plus six eight.
Equations which show the reactants and products as they are actually present in solution. What are the 3 types of ways to write chemical equations for reactions in solutions. Experts are tested by Chegg as specialists in their subject area.
Write the equations describing the electrode reactions and the net cell reaction for this electrochemical cell containing indium and cadmium. A balanced equation that represents an overall cell reaction is shown. Write a balanced equation to represent the picture shown using smallest whole-number ratios.
Write a balanced equation to represent the reaction shown. Their job side and you get the product is water and magic and gas. Sodium hydroxide ironII chloride sodium chloride ironII hydroxide.
7 Write a balanced equation to represent the reaction shown in the figure. The Na and O atoms are balancedone Na and one O on each sidebut there are two H atoms on the left and three H atoms on the right. I hope this is useful for you.
Write the coefficients as the lowest whole-number ratio. We review their content and use your feedback to keep the quality high. Usually us close fear No.
Discuss the common pollutants and the reaction that produces them. And that is the balanced chemical equation for the reaction shown in the figured attached. Write the coefficients as the lowest whole-number ratio.
Thus we need to increase the number of H atoms on the left. Experts are tested by Chegg as specialists in their subject area. Hydrogen oxygen water To the left of the arrow we have the before situation.
Use uppercase for the first character in the element and lowercase for the second character. 2 2 4. So the balanced chemical equation which represents the diagram provided in the question will be six X two plus six X.
This is a balanced chemical equation because 12 x and 12 5 molecules are present on the both sides of reactant and product. 3 molecules of CH4 and 3 molecules of N2Cl4. Write a balanced equation to represent the reaction shown.
So the balanced chemical Reaction representing the two diagrams will be 662 plus six. 1 2 2 1 4. Show through the use of stoichiometry how each pollutant could be reduced.
The chemical equation described in section 41 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. 4CH3 602 -4CO2 6H20 OCH4 602-3CO 6H2O O 3CH3 602-3CO2 6H20 O 3CH4 602 - 3CO2 6H2O. 4 4 yes.
This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Write a balanced equation to represent the overall reaction you would expect to occur in the electrolysis of aqueous NaBr.
Choose the cell notation which corresponds to the electrochemical cell that is described by this equation. View the full answer. Y represents the reactant While the product will be six x three right here here 18 X and six by molecules are present on the both sides that is reactant and product.
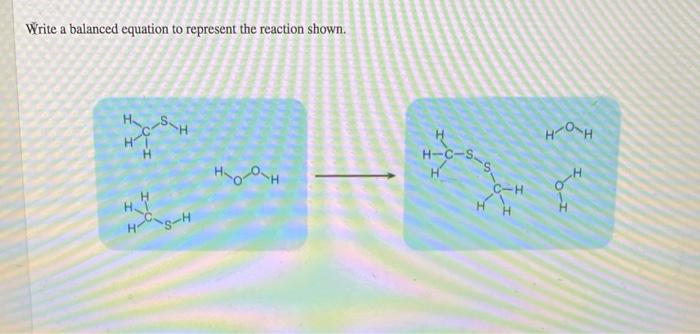
Solved Write A Balanced Equation To Represent The Reaction Chegg Com

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab
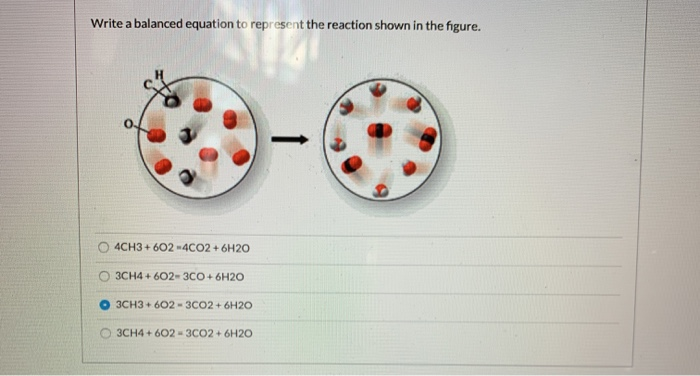
Solved Write A Balanced Equation To Represent The Reaction Chegg Com
No comments for "Write a Balanced Equation to Represent the Reaction Shown."
Post a Comment